

The Role of Caustic Soda in pH Adjustment: Its Mechanism and Importance in Industrial Processes
The Role of Caustic Soda in pH Adjustment: Its Mechanism and Importance in Industrial Processes
Let’s imagine a story unfolding at the heart of every industrial process; a tale of balance and precision. In the bustling world of factories and production lines, where every minor chemical reaction has a major impact on the final product, there is a key player, almost invisible: pH adjustment with caustic soda. This powerful substance is the silent hero that restores order and balance to industrial processes.
This topic isn't just a dry scientific discussion; it's the beating heart of many successful businesses. From water treatment plants that ensure public health to food production factories that bring flavor and quality to our tables, pH adjustment with caustic soda plays a vital role in every step. Today, we want to lift the veil on this secret and step into the complex world of this balance through a simple, narrative approach. Let’s explore together how this material helps industries operate at their best.
What is pH and Why Is It Important for Industry?
Before we dive into the solution, we need to fully understand the problem. pH is essentially a measure of how acidic or alkaline a solution is. The scale is graded from 0 to 14, where a pH of less than 7 indicates an acidic environment, a pH of more than 7 indicates an alkaline environment, and a pH of exactly 7 indicates a neutral environment. You might think this is just a simple number, but in the industrial world, a small digit can completely change the fate of a product or a process.
Imagine you own a cheese factory. If the milk's pH isn't adjusted correctly, it will have an undesirable texture, and the final product could spoil. Or in a paper mill, if the paper pulp's pH falls outside the ideal range, the paper's strength and quality will be severely compromised. This is where pH adjustment with caustic soda comes into play. It is a reliable solution for keeping the pH within the ideal range. If you're looking for buying caustic soda, you should know that this alkaline substance neutralizes excess acids and quickly restores balance. Thus, pH adjustment with caustic soda is a critical skill for production managers and process engineers.
The pH Adjustment Mechanism: A Simple Yet Powerful Chemical Reaction
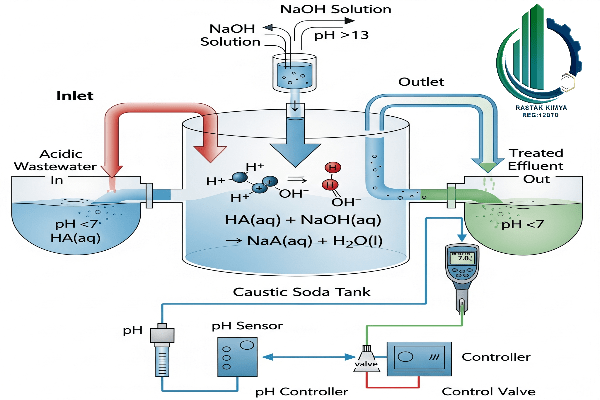
Now we get to the core of the matter. How can a white, solid substance have such a significant impact? The answer lies in the pH adjustment mechanism. Caustic soda, or sodium hydroxide (NaOH), is a strong base. When it dissolves in water, it breaks down into sodium ions (Na+) and hydroxide ions (OH−). These hydroxide ions (OH−) are the heroes of our story.
If a solution is acidic, it means the concentration of hydrogen ions (H+) is high. When we combine caustic soda and acid, the hydroxide ions (OH−) from the caustic soda quickly react with the hydrogen ions (H+) from the acid to form water (H2O). This reaction effectively reduces the concentration of hydrogen ions, and as a result, the pH of the solution shifts toward alkaline or neutral. pH adjustment with caustic soda utilizes this simple but incredibly effective mechanism.
This process clearly demonstrates the role of soda flake in neutralization. It not only neutralizes an acid but also eliminates potential hazards by converting it into salt and water. pH adjustment with caustic soda is a precise and efficient tool for controlling chemical balance.
A Success Story: When a Factory Found Its Balance
Let’s tell a story about a hypothetical water treatment plant. The manager, Mr. Hosseini, was facing a serious problem. The incoming water had a different acidity level every day due to weather conditions and industrial pollution. These pH fluctuations were damaging the expensive treatment equipment and reducing the quality of the outgoing water. He was in search of a reliable solution.
After a thorough review, the engineers at Rastak Kimia suggested pH adjustment with caustic soda. They installed an automated system that precisely added the necessary amount of soda flake as soon as the water’s pH dropped. The result was phenomenal. The water's pH was consistently maintained in the 7 to 8 range, damage to the equipment stopped, and the quality of the outgoing water reached its highest level. This is just one example of the success stories that have been made possible through the intelligent use of pH adjustment with caustic soda.
A Vital Role Across Industries: From Water to Food
The importance of pH adjustment with caustic soda in various industries is undeniable. Here are some of the most significant ones:
Water and Wastewater Treatment Industries
In this industry, pH adjustment with caustic soda is essential for neutralizing acids and removing heavy metals from the water. This process ensures the water is safe for human consumption or for being returned to the environment.caustic soda, as a strong base, easily brings the pH to the desired range and aids in the coagulation process.
Food Industries
In the production of many food products, including vegetable oil, chocolate, and dairy products, pH must be carefully controlled. pH adjustment with caustic soda helps remove impurities, prevent the growth of harmful bacteria, and improve the final product's flavor and texture.
Paper and Pulp Industries
In the paper production process, pH adjustment with caustic soda is crucial for separating cellulose fibers from lignin in the paper pulp (the Kraft process). This process significantly increases the paper's strength and quality.
Detergent and Textile Industries
In the production of soap and detergents, pH adjustment with caustic soda plays a central role in the saponification process. Also, in the textile industry, caustic soda is used for the mercerization of cotton, which leads to increased strength and luster in the fabric.
Soda Flake vs. Liquid Soda: Which is Right for You?
When choosing your caustic soda, you have two main options: soda flake and liquid soda. The difference between flake and liquid caustic soda is their physical form. Soda flake is a white, soli






